Second Opinion® 3D expands Pearl’s industry-leading AI capabilities to CBCT imaging—solidifying its position as the most advanced dental AI platform in the world
Pearl, the global leader in dental AI solutions, today announced that it has received FDA 510(k) clearance for Second Opinion® 3D, making Pearl the first and only dental AI company with FDA-cleared solutions for both 2D and 3D dental radiologic image analysis.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250527510723/en/
Pearl Becomes First Dental AI Company Cleared by FDA for Both 2D and 3D Imaging
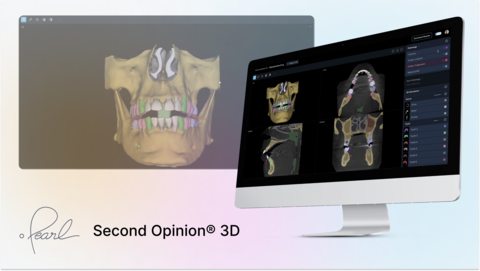
With this new clearance, Pearl’s Second Opinion® platform extends its AI capabilities to cone beam computed tomography (CBCT) imaging, enabling automated identification of critical anatomical structures—including dentition, maxilla, mandible, inferior alveolar canal and mental foramen (IAN), maxillary sinus, nasal space, and airway—in 3D scans.
“Pearl’s mission has always been to deliver the most advanced and clinically trusted AI solutions in dentistry,” said Ophir Tanz, founder and CEO of Pearl. “Becoming the first company to achieve FDA clearance for both 2D and 3D radiologic analysis isn’t just a milestone for us—it’s a milestone for dentistry.”
Second Opinion® 3D is designed to assist dental professionals in reviewing CBCT scans with greater speed, accuracy, and clarity. It empowers clinicians with instant AI-powered visualizations that support more precise diagnostics and treatment planning across specialties, including implantology, orthodontics, oral surgery, and airway management.
The FDA clearance follows extensive bench performance testing, in which Second Opinion® 3D demonstrated high segmentation accuracy across all targeted anatomical structures. Dice Similarity Coefficient scores exceeded clinical thresholds for every category, confirming both safety and effectiveness.
This clearance builds on Pearl’s track record as a regulatory leader in dental AI. The company’s original Second Opinion® platform remains the most widely deployed FDA-cleared solution for chairside AI pathology detection in 2D radiographs. With the addition of 3D capabilities, Pearl now delivers the only FDA-cleared platform capable of supporting comprehensive radiologic review across both major dental imaging modalities.
About Pearl
Pearl is an AI-driven company committed to enhancing patient care in dentistry. Founded in 2019 by a team with decades of experience developing successful, enterprise-grade computer vision solutions, Pearl introduced the first-ever FDA-cleared AI capable of reading and instantly identifying diseases in dental x-rays. With regulatory clearance in 120 countries, Pearl's AI assists dentists in making precise clinical decisions and effectively communicating with patients, thereby transforming the dental care experience worldwide. As dentistry’s global AI leader, Pearl is committed to the ongoing innovation of robust, accessible AI tools that improve patient health outcomes and build greater trust in dental medicine. To request a demo, please visit hellopearl.com/getdemo
View source version on businesswire.com: https://www.businesswire.com/news/home/20250527510723/en/
Contacts
Media Contact
Kate Gundry
pearl@pluckpr.com
617-797-5174
